Key benefits at a glance
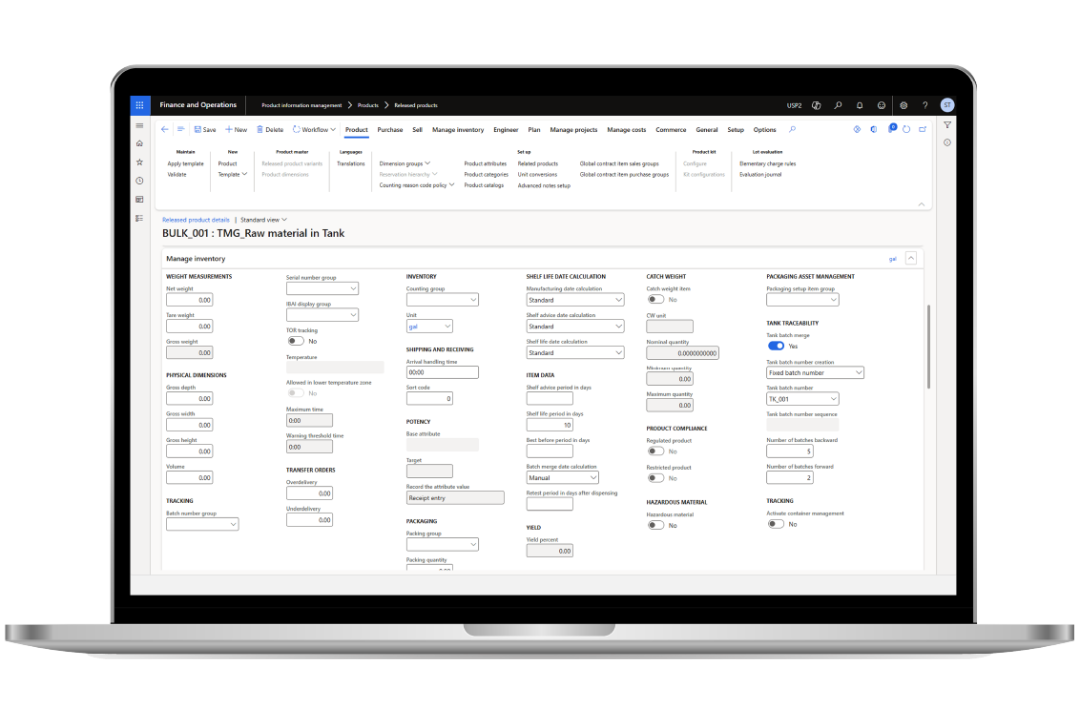
Optimize manufacturing operations
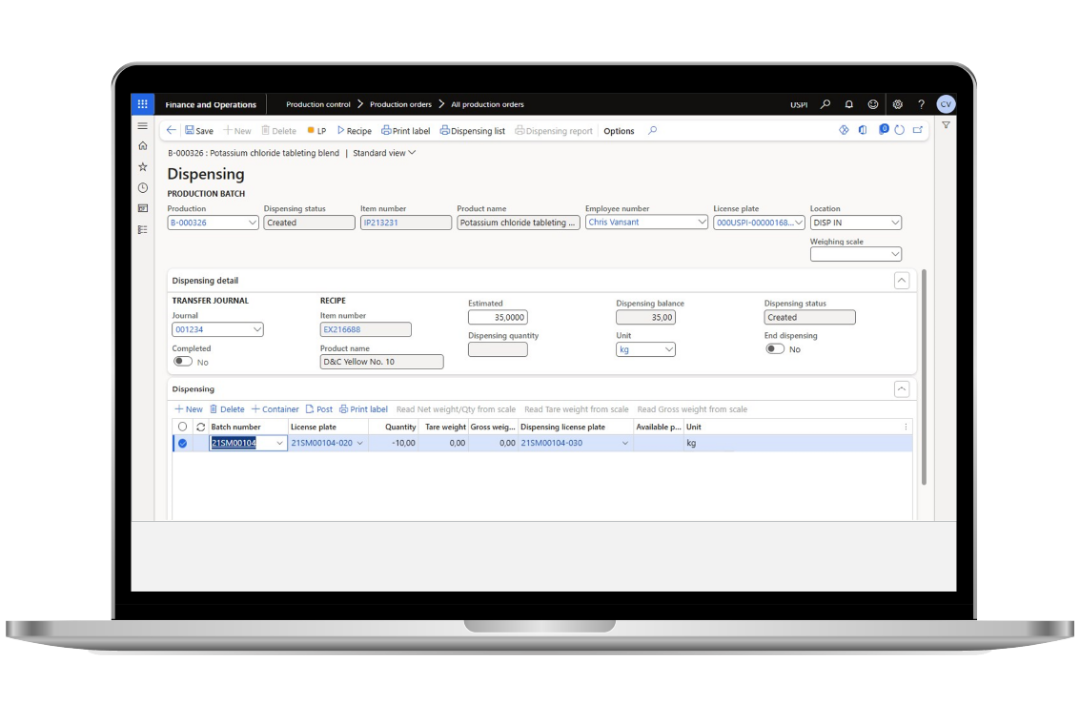
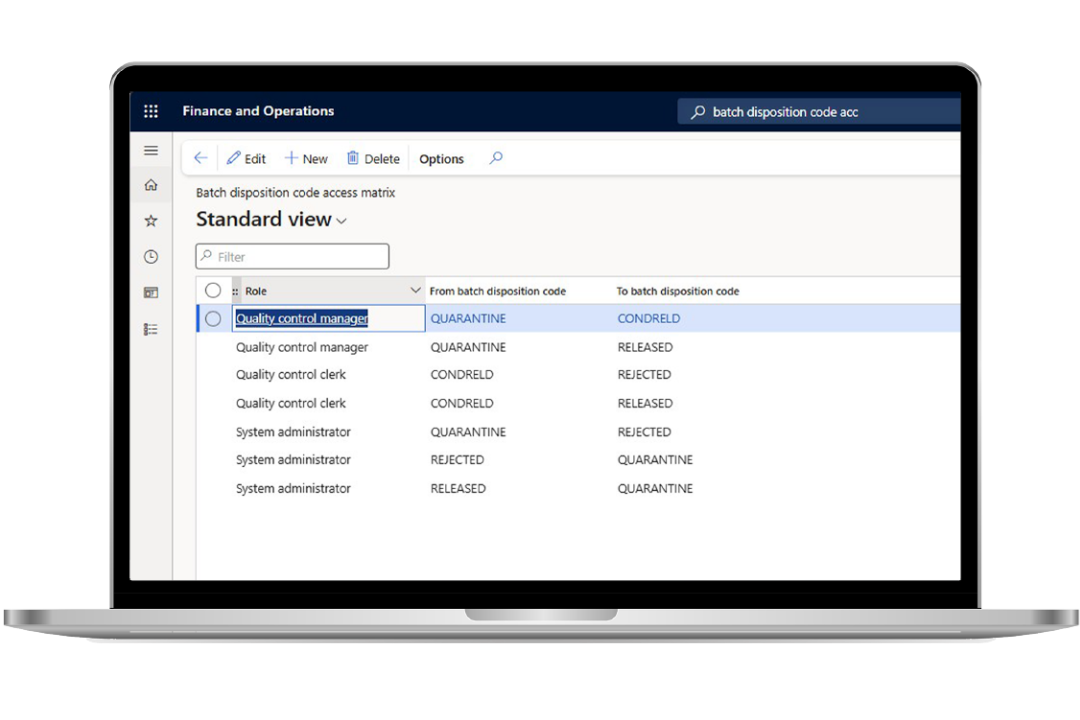
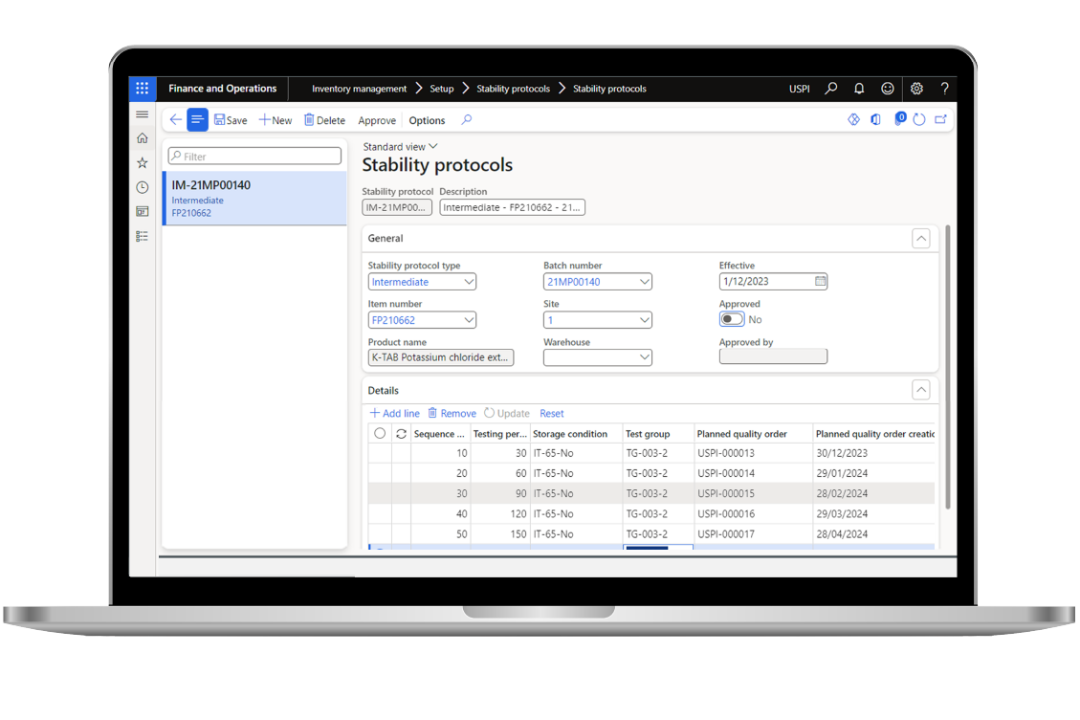
Improve quality control and quality assurance
Enhance lot and batch traceability
Ensure continuous compliance with global regulatory standards
Scale with a solution that grows with you





.png?width=150&height=150&name=Untitled%20design%20(59).png)
.png?width=150&height=150&name=Untitled%20design%20(57).png)
.png?width=150&height=150&name=Untitled%20design%20(60).png)
.png?width=150&height=150&name=Untitled%20design%20(56).png)